A research team led by Dr. Rihui Li, Assistant Professor at the Centre for Cognitive and Brain Sciences of the University of Macau (UM), and Dr. Allan Reiss, Professor at Stanford University, have achieved significant breakthroughs in understanding the neural mechanisms underlying Klinefelter Syndrome (KS). Utilizing advanced functional magnetic resonance imaging (fMRI) technology, the team has identified distinctive brain network alterations that correlate with pubertal development and behavioral impairment in boys with KS, offering new insights for tailored therapeutic interventions. The research has been published in European Child & Adolescent Psychiatry (IF = 6.0, JCR Q1), a prestigious journal in Psychiatry.
Klinefelter Syndrome, affecting approximately 1-2 in 1000 males, is a common sex chromosome disorder characterized by an extra X chromosome (XXY). Individuals with KS often exhibit a higher prevalence of neuropsychiatric conditions such as autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), and mood disorders, along with cognitive challenges, particularly in language skills. Understanding the neural basis of these symptoms has been a major focus of recent research.

Figure 1 Study design and the overall data analysis pipeline. (A) Participant recruitment and study data acquisition. A total of 46 boys with Klinefelter syndrome (KS) and a typically developing (TD, control) group of 51 boys were included in this study. Behavioral, clinical, and brain imaging data were collected from the participants. (B) Illustration of the overall data analysis pipeline.
In this study, Dr. Li’s team explored the association between resting-state brain networks and pubertal development and cognitive function in KS boys (Figure 1). The study included 46 KS boys and 51 age-matched typically developing (TD) boys, aged 8 to 17 years. The results revealed that KS boys exhibited widespread functional connectivity changes in brain regions such as the inferior frontal gyrus, temporoparietal junction, and hippocampus (Figure 2A-B). These regions showed increased low-frequency amplitude of fluctuation (fALFF) in motor areas and decreased regional homogeneity (ReHo) in the caudate nucleus (Figure 2C-D). Notably, these brain network alterations could accurately differentiate KS boys from TD boys and predict cognitive-behavioral difficulties in KS boys.

Figure 2. Altered brain network in males with Klinefelter syndrome (KS, n = 46) compared to the typically developing (TD, n = 51) group. (A) The matrix view of aberrant rsFC in boys with KS. (B) The aberrant rsFC within the brain network. (C) Increased (yellow) fALFF at the motor cortex. (D) Decreased (blue) ReHo at the left caudate (t = -4.898, P < 0.001) and right caudate (t = -5.110, P < 0.001) in boys with KS compared to the TD group.
Further, the study found that brain network changes mediated the relationship between total testosterone levels and language abilities, particularly in the functional connectivity between left angular gyrus-right hippocampus, right angular gyrus-right hippocampus, and left posterior middle temporal gyrus-right amygdala (Figure 3). These findings suggest that language learning difficulties are closely linked to pubertal developmental delays and may be regulated by key brain regions involved in executive function, learning, and emotional processing.
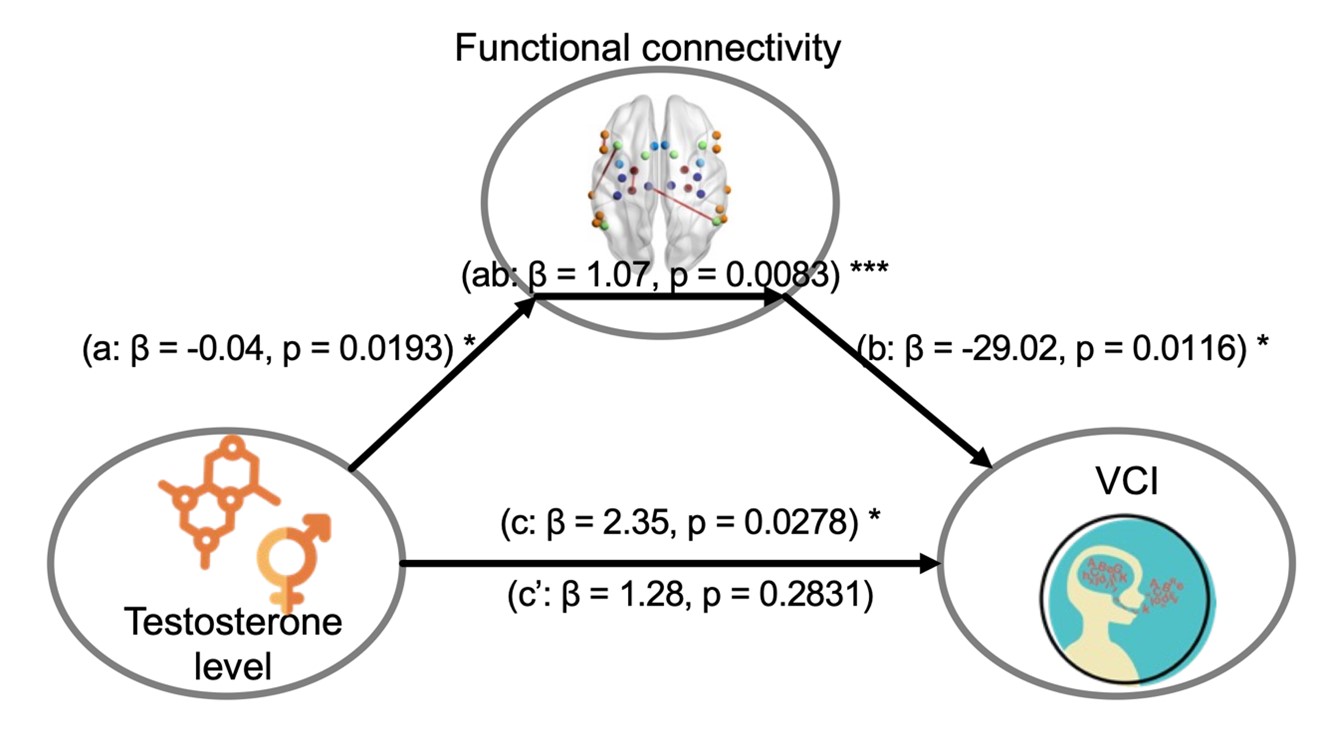
Figure 3. Analyses indicating associations between testosterone and VCI score are mediated by functional connectivity in the KS group (n = 37). a=the association of testosterone level with functional connectivity; b=the association of functional connectivity with VCI score; ab=the extent to which taking functional connectivity into account explains the association of testosterone level with VCI score; c=the association of testosterone level with VCI score; c’=the association of testosterone level with VCI score after controlling for the functional connectivity.
This groundbreaking research offers valuable insights into the neurobiological basis of cognitive and behavioral difficulties in individuals with KS, highlighting the interplay between pubertal development, brain function, and behavioral outcomes. These findings pave the way for developing targeted therapeutic strategies for KS boys and provide preliminary evidence on how testosterone deficiency and supplementation might impact behavior.
Prof. Rihui Li is the first and corresponding author of the research, with Allan Reiss, Professor at Stanford University, as the senior author. This research was supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01-HD092847), the University of Macau (SRG2023-00015-ICI), the National Natural Science Foundation of China (82301743), and the Science and Technology Development Fund of the Macao SAR (0010/2023/ITP1).
The full version of the article can be accessed at: https://doi.org/10.1007/s00787-024-02501-y


