A team led by Prof Zhen Yuan at Centre for Cognitive and Brain Sciences with the University of Macau (UM) has made significant progress in the treatment of Acinetobacter baumannii (A. baumannii) induced meningitis. The related research findings have garnered significant attention in the field of neuroscience and were just published in the internationally renowned journal Science Advances (Science sister journal with IF=13.6).
In recent decades, the excessive use of antibiotics has led to the emergence and widespread dissemination of multidrug-resistant bacteria, posing a significant challenge to public health. Among these multidrug-resistant bacteria, a few have been classified by the WHO as ESKAPE pathogens, requiring priority attention. One such pathogen is multidrug-resistant A. baumannii. To date, the availability of effective antibiotics for treating A. baumannii bacterial infections is very limited, leaving healthcare professionals with few options, known as the last line of defense – such as tigecycline and colistin. Therefore, the dire shortage of antibiotics necessitates immediate action to develop new antibiotics for the treatment of A. baumannii induced meningitis.
Meningitis is an infection and inflammation of the fluid and membranes surrounding the brain and spinal cord. In particular, the emergence of A. baumannii induced meningitis in this setting has caused a therapeutic challenge. To combat multidrug-resistant A. baumannii induced meningitis, the Yuan group carried out a comprehensive screening of over 1100 FDA-approved bioactive small-molecule drugs and inspected the efficacy of broxyquinoline (Bq) in combination with various metal ions. Among the examined compounds, the research team identified a metal complex named Zn(Bq)2, which was determined to be an exceptional candidate, exhibiting an ultra-low minimum inhibitory concentration of approximately 0.21μg/mL against A. baumannii. In particular, to overcome these limitations and ensure targeted delivery and action of the drugs, the research team used a series of innovative approaches. Firstly, outer membrane vesicles (OMVs) with selective targeting capabilities for A. baumannii were engineered through genetic modification. These OMVs can serve as carriers, delivering antibacterial metal complexes directly into the targeted infection areas of meningitis, thereby enhancing their efficacy. Secondly, nanoscale zeolitic imidazolate frameworks-8 (ZIF-8) were utilized as drug carrier platforms. The ZIF-8 framework allows for efficient loading and transport of the potent antibacterial metal complex Zn(Bq)2. By utilizing ZIF-8, the stability and bioavailability of the metal complex were enhanced, ensuring its efficient delivery to the targeted meningitis lesion. Through these integrated strategies, the research team successfully achieved a 99.9999999% inactivation of A. baumannii and a cure for A. baumannii-induced meningitis.
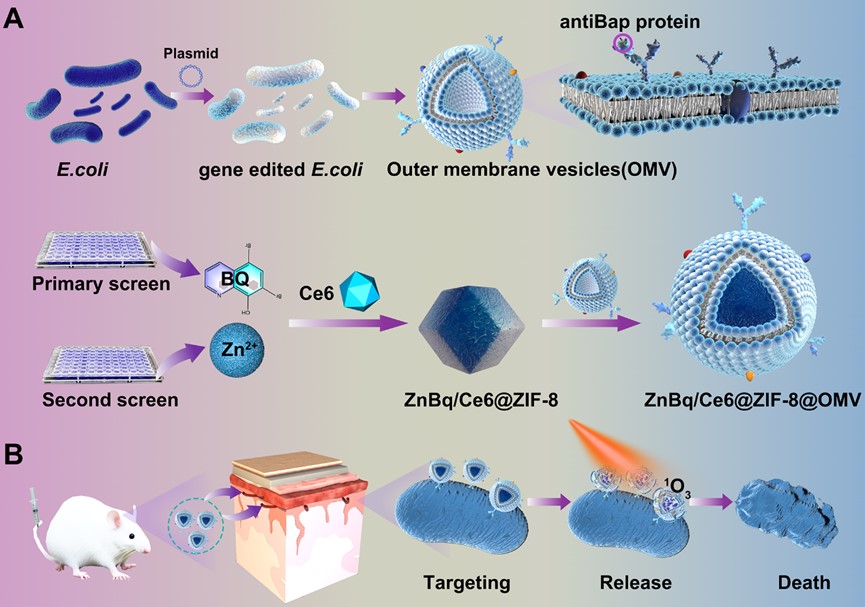
Schematic of construction of ZnBq/Ce6@ZIF-8@OMV for the treatment of A. baumannii induced meningitis. (A) Synthesis process of ZnBq/Ce6@ZIF-8@OMV. (B) Schematic of the antibacterial performance of ZnBq/Ce6@ZIF-8@OMV for targeted treatment of MDR A. baumannii-induced meningitis.
This study not only addresses the limitations of traditional antibiotics against A. baumannii but also provides a targeted strategy with genetically engineered OMVs, representing a significant advancement in the field of targeted treatment of A. baumannii-induced meningitis.
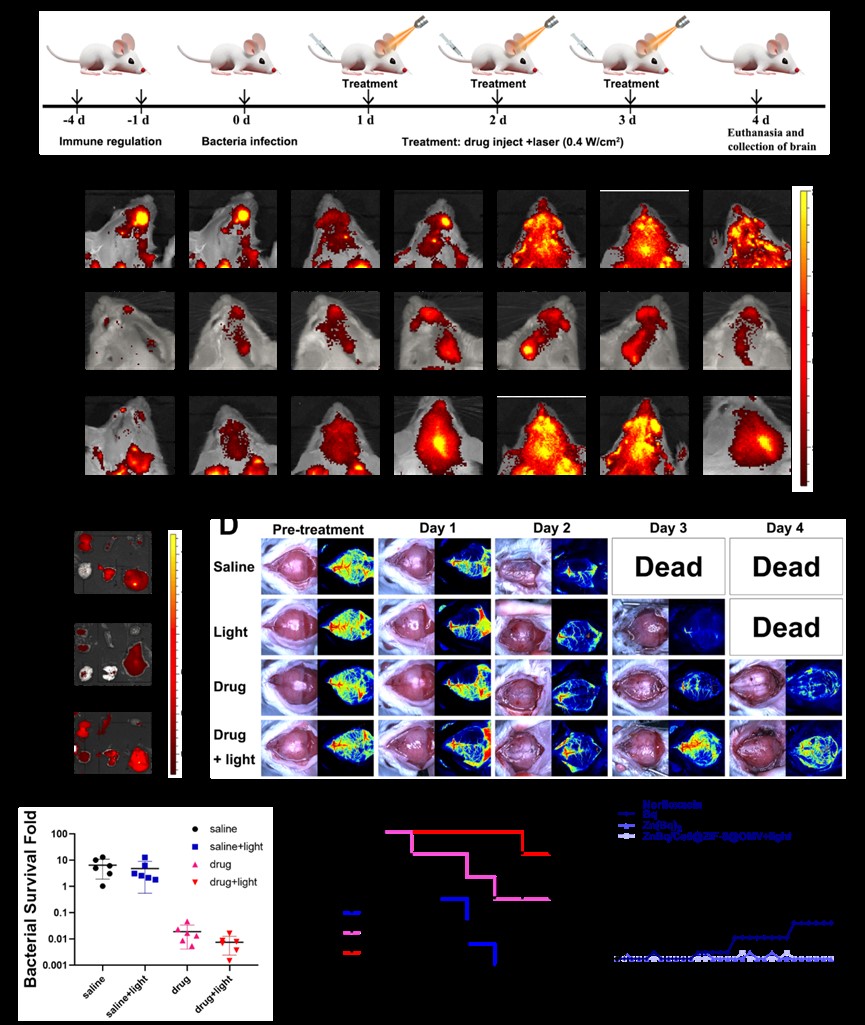
Efficacy of ZnBq/Ce6@ZIF-8@OMV in the treatment of A. baumannii induced meningitis in vivo. (A) Schematic of the construction and treatment process of meningitis infection. (B) Representative in vivo fluorescence imaging of BALB/c mice treated with ZnBq/Ce6@ZIF-8, large size ZnBq/Ce6@ZIF-8@OMV, and ZnBq/Ce6@ZIF-8@OMV at 0, 1, 2, 4, 6, 12, and 24 h. (C) Ex vivo fluorescence images of major organs at 24 h were shown. (D) In vivo photoacoustic imaging of meningitis mice with different treatment groups. (E) Bacteria content analysis of the brain tissue for different treatment groups. (F) Survival curves of infected BALB/c mice for various treatment groups. (G) Drug resistance assessment for different treatment groups.
The corresponding author of this study is Professor Yuan Zhen, and his PhD student Xianyuan Wei and previous postdoc Xue Bin as co-first authors of this paper equally contributed to this work. Prof. Ruan Shuangchen from Shenzhen Polytechnic University also offered financial support to this study. This project was funded by the Science and Technology Development Fund of Macau Special Administrative Region (File Nos. FDCT 0020/2019/AMJ and FDCT 0048/2021/AGJ) and the University of Macau (File Nos. MYRG2020-00067-FHS, MYRG2019-00082-FHS, MYRG2022-00054-FHS, and MYRG-GRG2023-00038-FHS). The full version of the research article and special report can be found respectively at https://www.science.org/doi/10.1126/sciadv.adk6331.


