Prof. Cheng-Teng Ip, Assistant Professor of CCBS, and her research team have conducted a phamco-electroencephalographic (EEG) study investigating signal complexity as a potential biomarker for predicting treatment response to ketamine in patients with major depressive disorder (MDD). Their findings, recently published in the Journal of Affective Disorders (five-year impact factor: 5.4), provide new insights into how subanesthetic doses of ketamine modulate brain dynamics and their implications for personalized treatment in depression.
Depression, particularly treatment-resistant depression (TRD), remains a debilitating condition that challenges conventional pharmacological approaches. Ketamine, an NMDA receptor antagonist, has demonstrated rapid and robust antidepressant effects in TRD, primarily through its modulation of neuroplasticity and glutamatergic signaling. However, the variability in treatment outcomes highlights the urgent need for neurobiological markers to help predict individual responses.
This study employed a placebo-controlled design and included 24 patients with major depressive disorder, of whom 21 were TRD patients. Treatment response was defined as a reduction of 33% or more in the Montgomery-Åsberg Depression Rating Scale (MADRS) score 24 hours after ketamine treatment. Resting-state EEG with eyes closed was recorded at four treatment conditions: pre-infusion, start-infusion (after the first infusion), end-infusion (after the second infusion), and 24 hours post-infusion. The results revealed that ketamine significantly increased whole-brain complexity (LZC) during infusion compared to placebo, reflecting enhanced neural complexity consistent with its neuroplastic effects (Figure 1). This feature served as a predictive marker in a logistic regression model, achieving an area under the receiver operating characteristic curve (ROC-AUC) of 0.75—highlighting its potential as a predictive biomarker (Figure 2). No residual effects on LZC were observed 24h post-infusion, affirming the pharmapheutical nature of ketamine. Intriguingly, patients who showed larger increases in LZC during the second infusion experienced less symptom improvement the following day. This suggests that excessive complexity might indicate a dysregulation in excitation–inhibition balance, potentially undermining therapeutic efficacy. (Figure 3).
These findings offer valuable insight into the neurophysiological mechanisms underlying ketamine’s antidepressant effects and support the use of EEG complexity as a tool for treatment stratification in MDD, paving the way for more personalized and effective interventions.
Prof. Cheng-Teng Ip and Prof. Martin Brunovský (Ministry of Health of the Czech Republic and Charles University) are co–last authors of the study, with Prof. Ip serving as the corresponding author. Weng-Lam Chan, a master’s student in Prof. Ip’s research team, is the first author. Data collection for the study was supported by the Ministry of Health of the Czech Republic (NV18-04-00260) and the Charles University research program Cooperatio–Neurosciences. The project was funded by the University of Macau (SRG2023-00040-ICI and MYRG-GRG2024-00022-ICI).
The full article is available at: https://doi.org/10.1016/j.jad.2025.119477
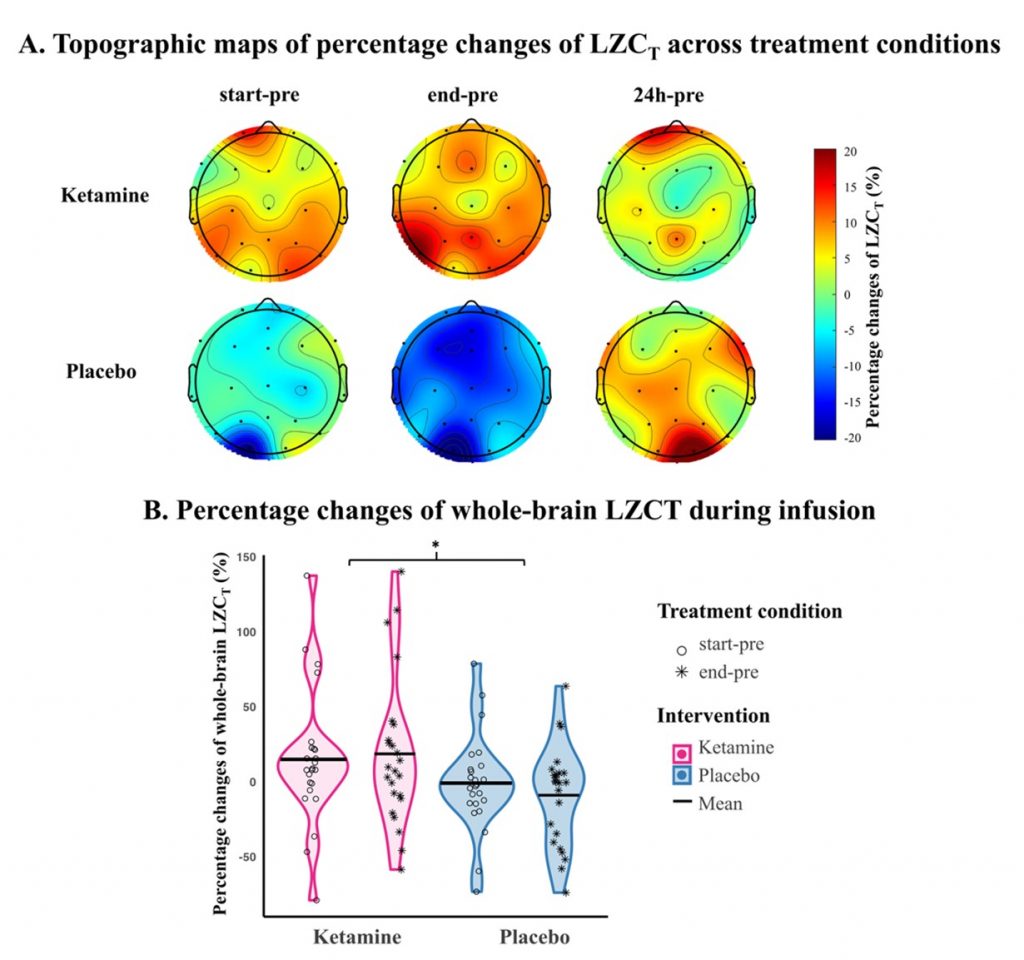
Figure 1. Effects of ketamine and placebo on LZCT.
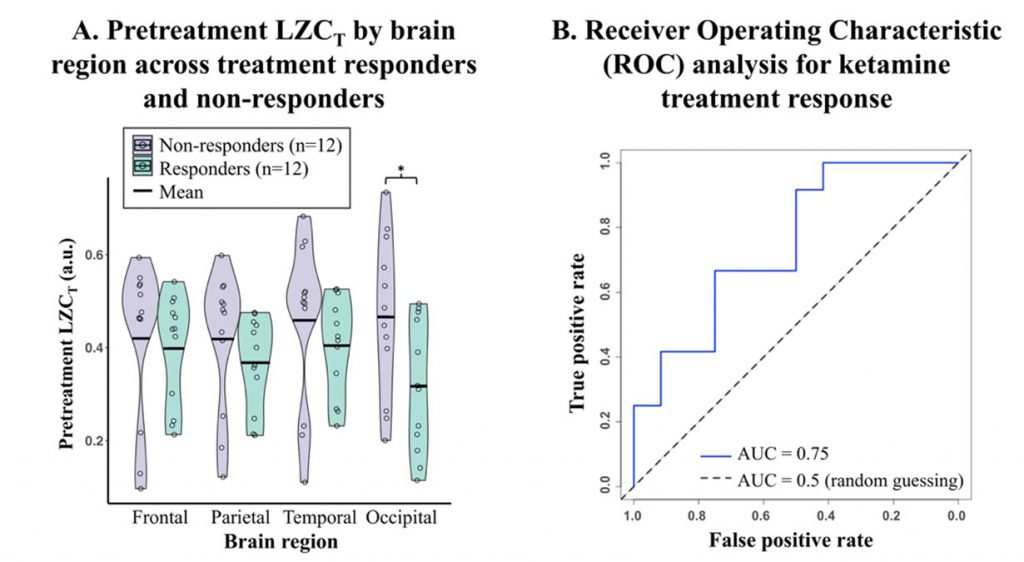
Figure 2. Occipital LZCT as an indicator of ketamine response.
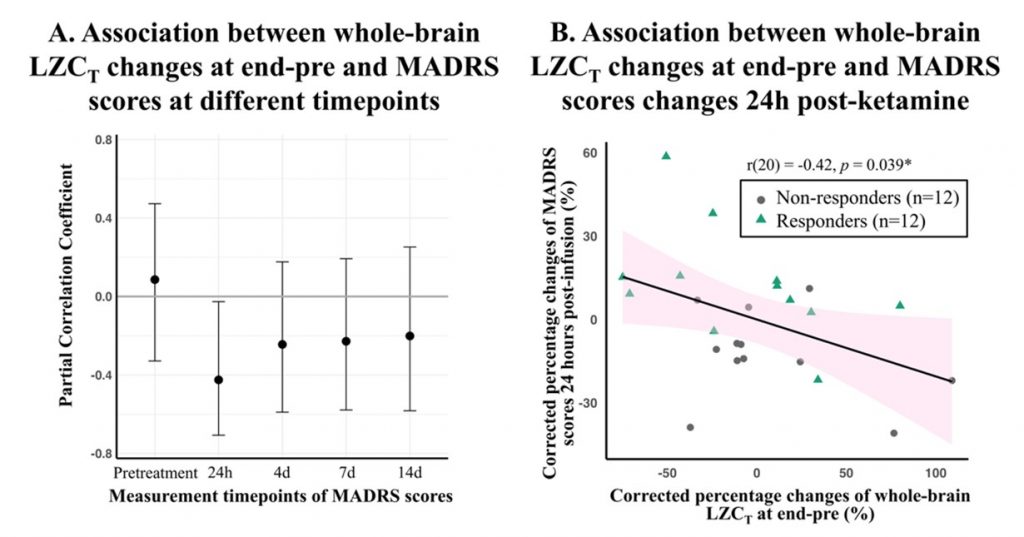
Figure 3. Whole-brain LZCT at end-pre in association with MADRS scores measured at pretreatment and various timepoints post-ketamine.


