A collaborative research effort led by Professor Rihui Li from the Centre for Cognitive and Brain Sciences and Professor Qiong Xu of the Children’s Hospital of Fudan University has uncovered distinct and shared patterns of brain network dysfunction in children with Autism Spectrum Disorder (ASD) and Fragile X Syndrome (FXS). Published in the prestigious journal Molecular Psychiatry (5-Year IF=11.8, JCR Q1), the research provides critical insights into the neural mechanisms underlying these neurodevelopmental disorders.
ASD, affecting 1-2% of children globally, is characterized by profound social communication deficits and repetitive behaviors. Its high heterogeneity has long challenged mechanistic research. The study confronts this complexity by focusing on FXS, the most common monogenic cause of ASD, stemming from FMR1 gene CGG repeat expansion, as a genetically homogeneous subgroup sharing overlapping behavioral phenotypes with idiopathic ASD.
Utilizing resting-state functional MRI (fMRI) data from 70 children with idiopathic ASD (unidentified cause), 37 with FXS, and 43 typically developing (TD) controls, the team conducted advanced functional connectivity and network topology analyses.
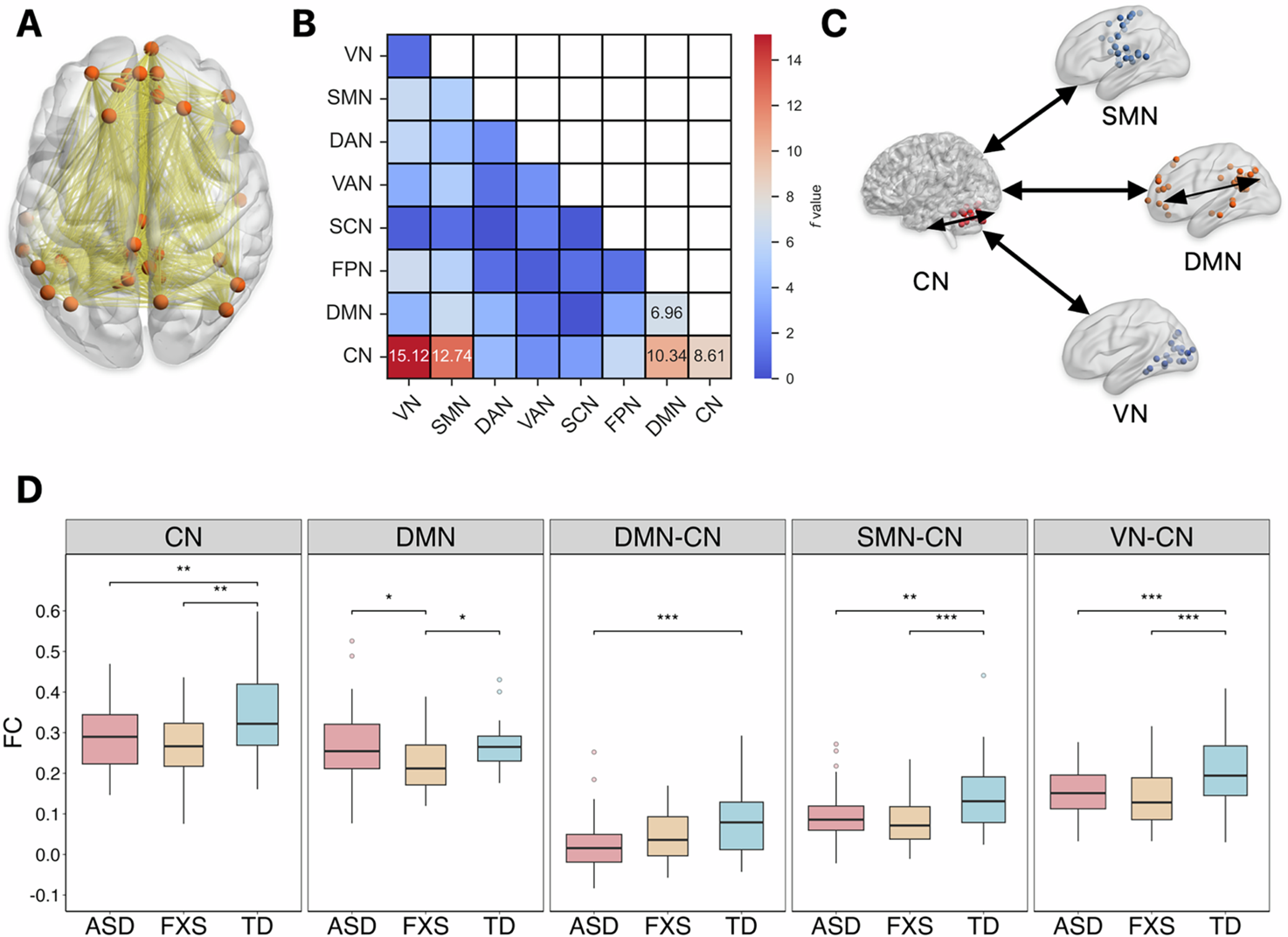
Children with FXS showed a unique brain change (Figure 1). Their Default Mode Network (DMN) had significantly weaker internal connections. The DMN is critical for self-referential cognition, memory processing, and emotional regulation. This impairment clearly separated FXS from both ASD and TD children. Both the ASD and FXS group had weaker connections within the Cerebellum Network (CN). Connections between the CN and other key networks were also weaker compared with TD, including connectivity of the CN-DMN, CN-Sensorimotor Network, and CN-Visual Network. This suggests a common neural substrate for core behavioral phenotypes. While both disorders showed aberrant topological properties, their abnormalities vary (Figure 2). ASD had uniquely reduced global and local efficiency in the whole brain network, and reduced clustering coefficient in cuneus, opercular cortex and precentral gyrus. FXS showed its own distinct pattern of reduced degree and clustering coefficient within the cerebellum.
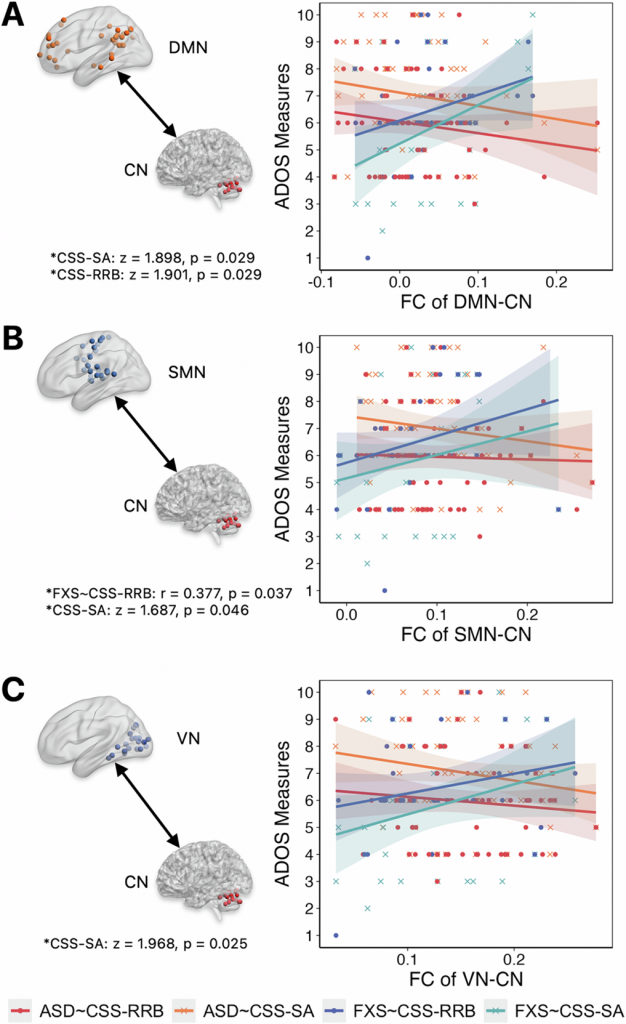
The study also found critical clinical differences (Figure 3). Neurobehavioral decoupling was observed in the correlation between the severity of social affect symptoms and several between-network connectivity for FXS versus ASD. Moreover, an epigenetic link was established in FXS. The degree of the cerebellum’s Crus I demonstrated a significant negative correlation with FMR1 gene DNA methylation levels. This latter finding provides direct evidence connecting macro-scale neuroimaging biomarkers to molecular disease mechanisms in FXS, opening new pathways for biologically stratified interventions.
This research establishes DMN suppression as a neural signature of FXS and identifies cerebellar dysfunction as a shared pathological foundation in FXS and ASD. The discovery of disorder-specific network-behavior relationships advocates for mechanistically targeted interventions. Future work will focus on expanding longitudinal designs tracking cerebellar FC alongside behavioral outcomes and developing targeted intervention therapies.
Original article link: https://www.nature.com/articles/s41380-025-03112-y
Prof. Rihui Li from the CCBS of the University of Macau, Prof. Qiong Xu and Prof. Zhongwei Qiao from the Children’s Hospital of Fudan University are the co-corresponding authors of the article. PhD student Danyong Feng from the CCBS of the University of Macau and Dr. Dongyun Li from the Children’s Hospital of Fudan University are the co-first authors of the article. This work was supported by grants from the National Natural Science Foundation of China (82171540, 82301743), the Science and Technology Development Fund of the Macao SAR (0010/2023/ITP1), the University of Macau (SRG2023-00015-ICI), Natural Science Foundation of Anhui Province (No.2308085MH255), grant funds of China Medical Board (CMB) (22–471), foreign expert program of Ministry of Science and Technology (G2022132004L), academic leaders development program of Children’s Hospital of Fudan University (EKXDPY202306).
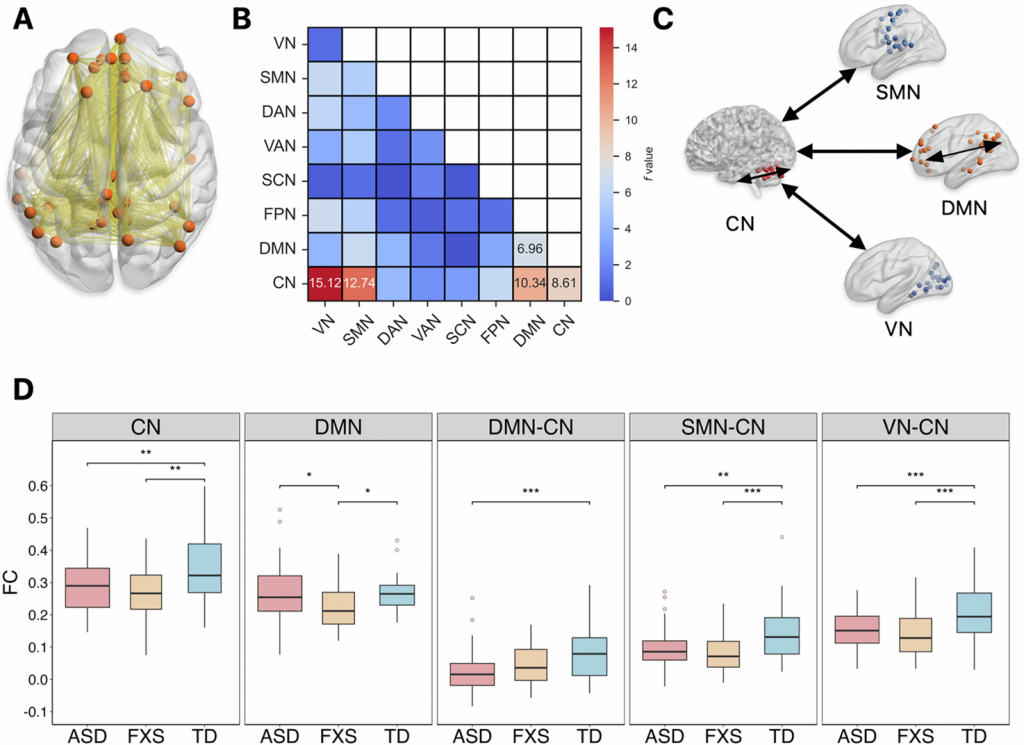
Figure 1. Comparisons of the mean FC within and between the intrinsic brain networks showed significant group differences.
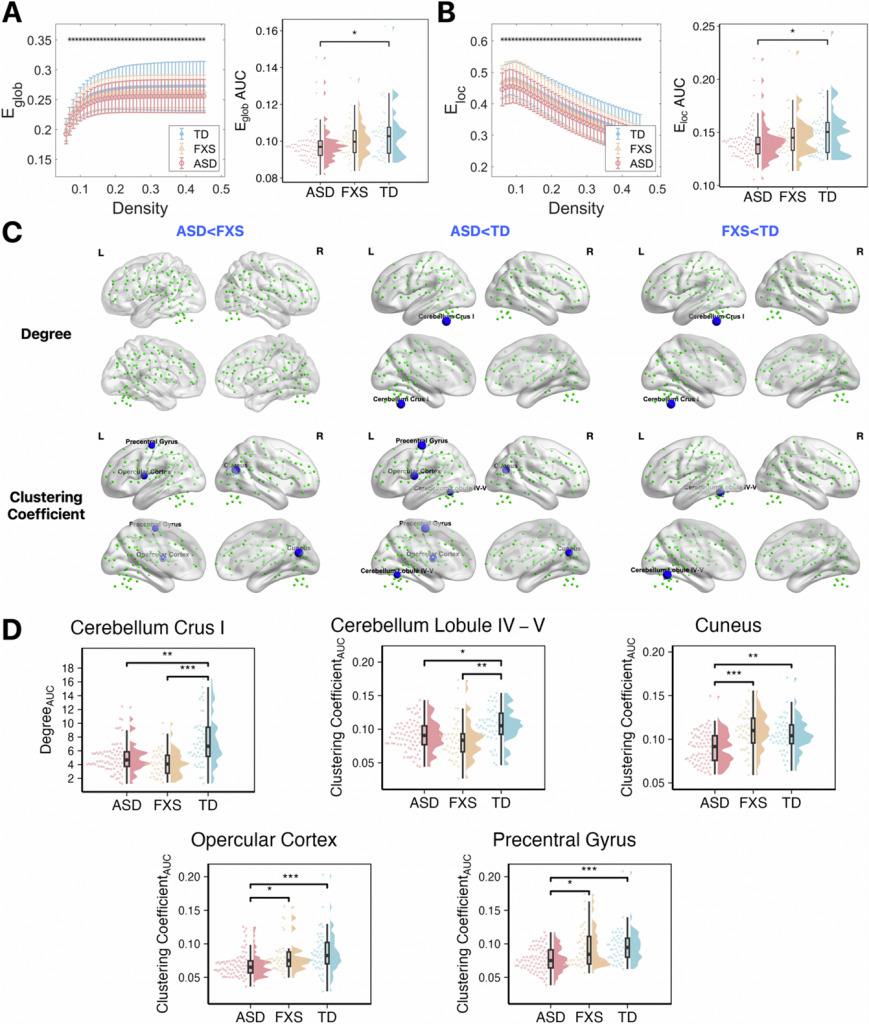
Figure 2. Group differences in the network topological properties.