[Please click this link for the University report.]
A groundbreaking study led by Professor Zhen Yuan with the Centre for Cognitive and Brain Sciences (CCBS)/Faculty of Health Sciences (FHS) at the University of Macau has uncovered dysfunction in the brain’s waste clearance system (glymphatic system) in early psychosis patients. This research provides a novel neuroimaging biomarker for early diagnosis and intervention in psychiatric disorders. This important finding was published in Molecular Psychiatry, one of the top international journals in neuroscience and psychiatry (5-year impact factor = 11.1, nature sister journal).
The brain’s glymphatic system plays a vital role in clearing metabolic waste and supporting neural health. Although glymphatic dysfunction has been implicated in neurodegenerative diseases like Alzheimer’s and Parkinson’s, its involvement in psychiatric disorders, particularly in early psychosis, remains unclear.
In this study, Prof. Yuan’s group inspected the multimodal MRI neuroimaging data from 137 young participants (ages 16–35). The cohort consisted of healthy controls, individuals with non-affective psychosis, and those with affective psychosis. In particular, the team measured the coupling between cortical activity (BOLD signal) and cerebrospinal fluid flow (CSF signal), a biomarker for the brain’s waste clearance efficiency.
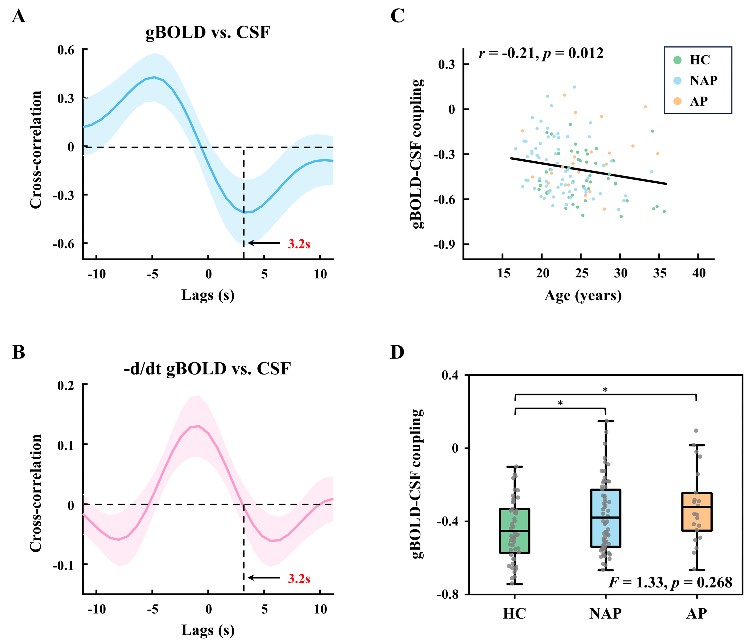
Fig. 1. Global BOLD-CSF coupling strength is associated with age and varies significantly between different groups.
Notably, these dysfunctions were most pronounced in high-order cognitive networks including the default mode and frontoparietal networks, whereas lower-order sensory and motor regions (e.g., visual and somatomotor networks) remained relatively intact. This pattern suggests a “top-down” hierarchy of impairment in early psychosis, with higher cognitive systems being disproportionately affected early in the disease process (Fig. 2).
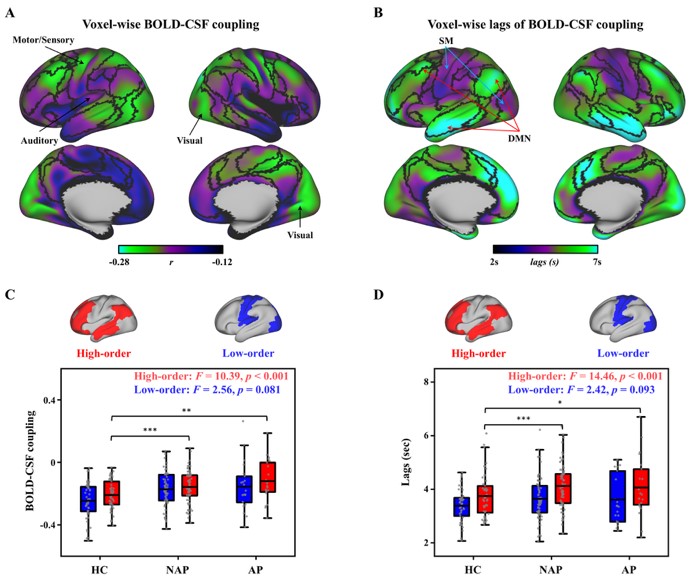
Fig. 2. The coupling strength and time lags between voxel-based BOLD and CSF signals.
Critically, the study confirmed that the BOLD-CSF coupling index exhibited high stability and reproducibility across multiple independent datasets, enhancing its reliability across different platforms and populations (Fig. 3). This robustness establishes BOLD-CSF coupling as a compelling neuroimaging biomarker for psychiatric disorders, which can enhance early detection, track disease progression, and assess therapeutic response.
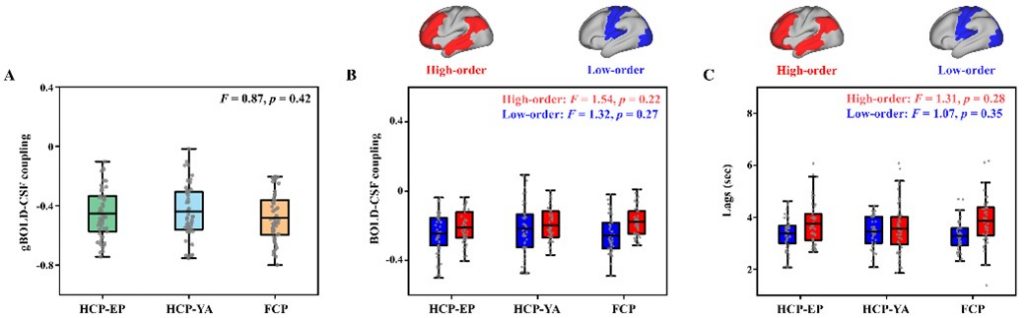
Fig. 3. Reproducibility of BOLD-CSF coupling across independent datasets.
The work was mainly performed by Dr. Lin Hua (a former PhD student now works as a postdoctoral fellow at Harvard Medical School) under the supervision of Prof. Zhen Yuan. Prof. Yuan’s former lab member, Dr. Zhiying Zhao also provided some help for this work. The research was supported by the Macao Science and Technology Development Fund (FDCT 0014/2024/RIB1 and FDCT 0015/2023/ITP1) and the University of Macau (MYRG-GRG2023-00038-FHS and MYRG-GRG2024-00259-FHS).
Original article link:


